Your heart is a tireless organ that beats about 3 billion times over an average lifetime and is simply essential for life. Unsurprisingly, cardiovascular disease is the leading cause of death worldwide, costing millions of lives each year. This relentless condition primarily damages the heart, which is divided into four main chambers: the right atrium, left atrium, right ventricle, and left ventricle. Understanding the functions and vulnerabilities of these chambers is crucial in the fight against heart disease.
Imagine your heart as a house with four rooms. The right atrium is your heart's entryway, receiving oxygen-poor blood from the body, and the right ventricle is the pump that guides the blood in. The left atrium receives oxygen-rich blood from the lungs, and the left ventricle pumps the oxygen-rich blood back out to the rest of your body, supplying your organs and tissues with the oxygen they need to function.
The right atrium, your heart’s entryway, is a critical area, and also houses your heart's pacemaker region, known as the sinoatrial (SA) node, which acts as your heart's natural clock. It generates electrical impulses that regulate the heartbeat, ensuring a steady rhythm. Any problems in the right atrium can lead to irregular heartbeats, known as arrhythmias, and ultimately lead to a heart attack.
Despite the right atrium's importance, it has remained overlooked, until now.
Single-cell profiling of an aching heart
A new study from researchers at MIT and collaborators highlights how heart disease affects the right atrium, and reveals how it changes at single-cell resolution across various heart diseases, along with the pericardial fluid surrounding the heart.
The researchers collected precious samples from open heart surgeries in different pathological stages, ranging from reduced blood flow to the heart (ischemic heart disease), heart attack (myocardial infarction), and ultimately heart failure.
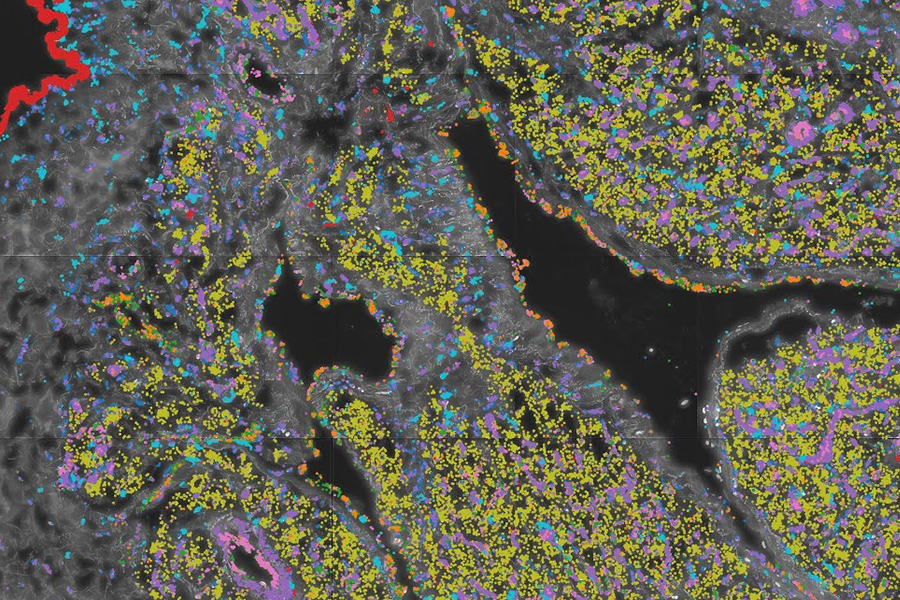
The study used state-of-the-art microfluidic techniques and high-throughput sequencing to isolate individual cells from heart tissue samples, tagging each RNA molecule with the unique cell it came from, and enabling researchers to see the unique gene expression profile of 300,000 cells across dozens of donors.
The researchers used these datasets to annotate 60 cell types from eleven populations and used them to understand how heart disease progresses, and how different cells and different cell types interact. This resulted in the most detailed map of the heart's cell types and their functions across different disease states.
A complex architecture
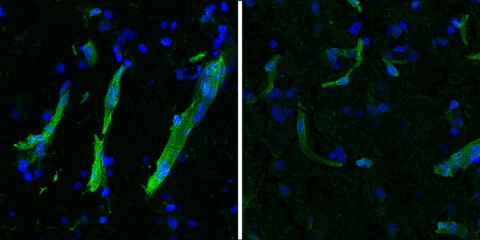
The heart contains a dense network of contractile muscle cells called cardiomyocytes, which work tirelessly to keep the heart beating. These cells are supported by a complex network of microvessels essential for delivering oxygen and nutrients. Cardiomyocytes have the highest number of mitochondria among all human cells, reflecting their high energy demands. This complex architecture requires an extensive network of blood vessels to supply nutrients and oxygen while removing metabolic waste and CO2.
The study revealed how ischemic heart disease damages this structure at the molecular level, and the cell types and genes that are likely to be ultimately responsible for the loss of oxygen supply for the cardiomyocytes. Therefore, it is crucial to study the vessels themselves. This study has extensively characterized their subtypes and specific gene expression profiles at high resolution. Additionally, the heart's intricate architecture necessitated advanced high-resolution spatial analysis, which provides precise localization of gene expression within the tissue. In turn, this adds another dimension to understanding the signals of interest.
Immune players
These findings highlight the importance of investigating all parts of the heart to understand what drives severe cardiovascular disease. The study reveals metabolic changes in the right atrium and identifies molecules responsible for widespread inflammation. While inflammation is the body's response to injury or infection, chronic inflammation can cause damage and trigger harmful changes in heart tissue. Targeting these inflammatory molecules could reduce inflammation and prevent serious heart damage. Notably, the single-cell resolution of the dataset enabled the identification of specific immune cell populations and alterations in their composition and gene expression profiles across different disease stages. This suggests that these immune cell populations contribute to the widespread inflammation observed in cardiovascular disease.
Pericardial fluid
Pericardial fluid is a vital, clear liquid that fills the space between the heart and the pericardium, a protective sac surrounding the heart. This fluid serves as a cushion, reducing friction between the heart and the pericardium as the heart beats. Moreover, pericardial fluid holds significant potential as a biomarker. Biomarkers are biological indicators used to detect or predict disease states. Studying pericardial fluid can reveal specific proteins, cells, or other substances that indicate the presence of heart disease. Pericardial fluid biopsies are also considered less invasive than cardiac biopsies, highlighting the interest in better understanding its composition. This study was the first to analyze gene expression at a single-cell resolution from pericardial fluid samples, revealing unprecedented details in its cellular composition and transcriptomic profiles. Most cells found were immune cells, showing a large overlap with the immune cells present in heart tissue itself. The study also revealed major transcriptomic changes associated with diseases. Spatial analyses of heart tissues also revealed a concentration of immune cells in the layer of the heart that is in contact with the pericardial fluid, highlighting potential interactions.
The road to treatments
While treatments for ischemic heart disease and heart failure have improved over time, they primarily address the effects of the disease after it has occurred. These treatments manage symptoms and delay further damage to the heart. However, despite advances, the five-year survival rate for heart failure remains grim, often more deadly than many cancers. The ultimate treatment for end-stage heart failure is a heart transplant, highlighting the condition's severity.
There is a critical need for preventive measures to stop heart disease before it reaches advanced stages. This requires a deeper understanding of the underlying causes and precise molecular mechanisms of the condition. By uncovering how heart disease affects different parts of the heart, such as the right atrium, researchers can develop more effective strategies for prevention, early detection, and treatment.
An invaluable resource
The full dataset and interactive tools developed by the researchers are available online, offering valuable resources for further research into the genetic and molecular underpinnings of cardiovascular disease. These comprehensive findings mark a significant advancement in cardiovascular research, opening new avenues for therapeutic intervention and prevention strategies.